As a qualified aesthetics nurse, I can reassure all my clients that I am both fully trained in and only use genuine Dermaroller treatments.
![Dermaroller_0195[1]](http://www.beckyhollands.co.uk/wordpress/wp-content/uploads/2011/09/Dermaroller_01951-150x150.jpg) The safety and use of Skin needling devices is of frequent debate. The question is, does it matter if the skin needling device you purchase is certified an EU Medical Device? If it does, how can you distinguish a genuine, quality and safety assured device from just a cheap imitation or a device that has not been certified to the same standard? And lastly, how does the certification of the device affect your insurance as a practitioner, even if you are just carrying out cosmetic treatments?
The safety and use of Skin needling devices is of frequent debate. The question is, does it matter if the skin needling device you purchase is certified an EU Medical Device? If it does, how can you distinguish a genuine, quality and safety assured device from just a cheap imitation or a device that has not been certified to the same standard? And lastly, how does the certification of the device affect your insurance as a practitioner, even if you are just carrying out cosmetic treatments?
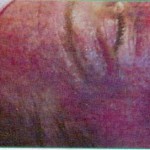
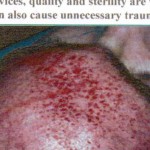
It becomes increasingly evident that unfortunately some companies and individuals see a derma roller purely as a cosmetic beauty tool. They disconnect it from the considerations normally associated with the use of needles i.e. of sterility. Whilst the outcome of a treatment is to improve the appearance of an individual’s skin, therefore a cosmetic outcome, using a device that contains needles that penetrate the skin carries a substantial health risk. To safeguard against possible infections or skin damage, it is therefore a priority to know what brand of derma roller you can trust.
There are a couple of key pieces of information which will help you to identify a derma roller that comes with a honest quality, sterility and therefore safety guarantee and one that just tries to convince you it does. The ambiguity is certainly not helped by the fact it is not currently a legal requirement for skin needling devices which are intended for use to assist cosmetic conditions, as opposed to treating therapeutic conditions, to undergo any form of independent inspection.
The crucial indicator is whether a derma roller is classified as an EU Medical Device Class IIa or not. To meet the requirements of this certification, a company’s manufacturing premises and products have been inspected and approved by an independent European notified body. As the EU notified body is answerable to the individual European government, stringent approval processes are exercised.
The pictures show the possible consequences of using inferior needle quality rollers
(Copyright Ferndale Pharmaceuticals LTD)
The second category of EU Medical Devices of relevance is Class I. It may be surprising to know, that in contrast, Class I Medical Devices and the facilities in which they are produced have undergone zero independent inspections. However, on the face of it, you might presume products to be of the same standard. The way to tell the difference is to look out for a 4 digit number that will follow the CE mark on the packaging. Class I EU Medical Device derma rollers will not have this unique set of the numbers which correspond to inspecting independent notified body. For White Lotus Anti Aging’s Lotus Roller derma roller for example, this is: 0197. To our knowledge, there are only 2 skin needling rollers in the world to be recognised as Class IIa EU Medical Devices under EU Medical Device Directive 93/42.
Adding to the already murky waters, are illegitimate skin needling devices (in terms of safety and quality assurance) that use FDA Approval, FDA Listed or FDA Registered on their packaging and in their marketing. This sounds very impressive, until you realise that this simply means they have self-regulated themselves as a Class I medical device. Basically they have filled out a form and paid a fee. At no point were their manufacturing premises inspected or did the FDA guarantee the sterility or quality of their product.
We anticipate these issues becoming increasingly important for practitioners wanting to gain insurance to practice skin penetration techniques in the future, as skin-needling and the fantastic results that entail continues to catch on in the wider beauty industry and as companies keen to capitalise on this demand continue to introduce dangerous, poor quality imitations, posing substantial risk to consumers.
White Lotus Anti Aging regularly campaigns to promote understanding in the industry and to consumers of what the differences in certifications are and danger signs to look out for, for the safety of everyone concerned. We hope we have clarified any misperceptions.